The LEDs (Light Emitting Diode) are diodes whose basic characteristic is the ability to emit light when they are passed through a current that flows from P to N region. At each recombination between the charge carriers (electrons and holes), on PN junction region, a photo emission is generated, and the total quantity of emitted photons, and therefore the light intensity, is proportional to the current intensity that passes through them. The emitted light has a spectrum – wavelengths distribution – that is defined according to the materials used in the realization of the diode PN junction, although it partially depends on the current intensity and on the junction temperature. The most common materials, used in LEDs production, are those belonging to the III° and V° group of the elements periodic table:
• Gallium arsenide (GaAs) for light from infrared to red (650 nm);
• Gallium arsenide and phosphate (GaAsP) for light from red to yellow (630-590 nm);
• Gallium phosphate (GaP) for wavelength from blue to green (565 nm);
• Gallium nitride (GaN) for blue light (430nm);
• Indium and Gallium nitride (InGaN) for the deep blue until to ultraviolet (390 – 360nm);
The white LEDs are realized and through LED combination of the three RGB basic colours and using blue LED covered with a semi-transparent layer of yellow emitting phosphorus.
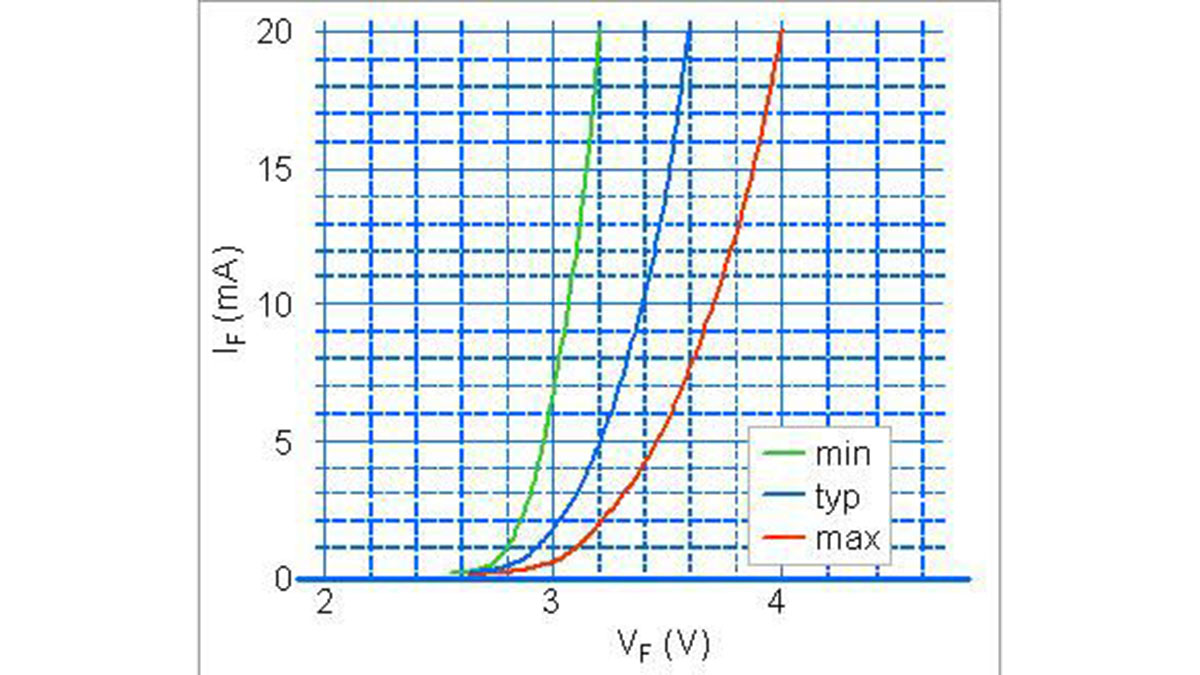
2. Voltage/Current Features
LEDs have a very similar behaviour to the standard diodes and they have a VF direct voltage fall joined to the IF direct current as shown in the Fig. 1. The VF direct voltage depends on LED realization technology and it is about 1,4 – 2V for the GaAs, 2 – 2,5 V for the GaAsP, 3 – 3,5 V for the GaP e 3,8 – 4,5 fV or the InGaN (blue or white LEDs).
For More Details: Driving LEDs
